- cross-posted to:
- science@lemmy.ml
- cross-posted to:
- science@lemmy.ml
As I said over on science@lemmy.ml
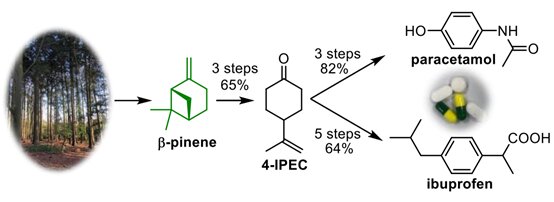
This is super cool! And they were able to make another major chemical precursor too. I see the yield for ibuprofen is only 64% but I have to assume that’s there room to optimize that reaction scheme to generate higher yields!
I would love to see the full synthesis process as well as reaction conditions, but like everyone else who leaves academia I lost access to journals after finishing my grad program.
I noticed the PDF is available if you simply throw the article title into scholar.google.com .
Edit: Wait, it is just open-access. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202300670
That’s awesome! Kudos to the European Chemical Society, ACS loves their paywall
but like everyone else who leaves academia I lost access to journals after finishing my grad program.
I still have my academic account but the sci-hub experience is so much easier that I just use that anyway.
They successfully converted β-pinene into two everyday painkillers, paracetamol and ibuprofen, which are produced on ~100,000 ton scales annually.
At first I couldn’t believe that we make 100,000 TONS of paracetamol (acetaminophen) a year.
1E5 tons per year / 6E9 people on earth = 17 grams per person per year
My tylenol pills are 500mg, and ~34 pills per year seems about right.
Wow. At a density of 1.3 g/cm^3 that’s about half an olympic swimming pool of acetaminophen a year.
Nice breakdown. Maybe update your stats a little, we’re at nearly 8e9 people on the planet. Doesn’t change your conclusion much.