- cross-posted to:
- health@lemmy.world
- cross-posted to:
- health@lemmy.world
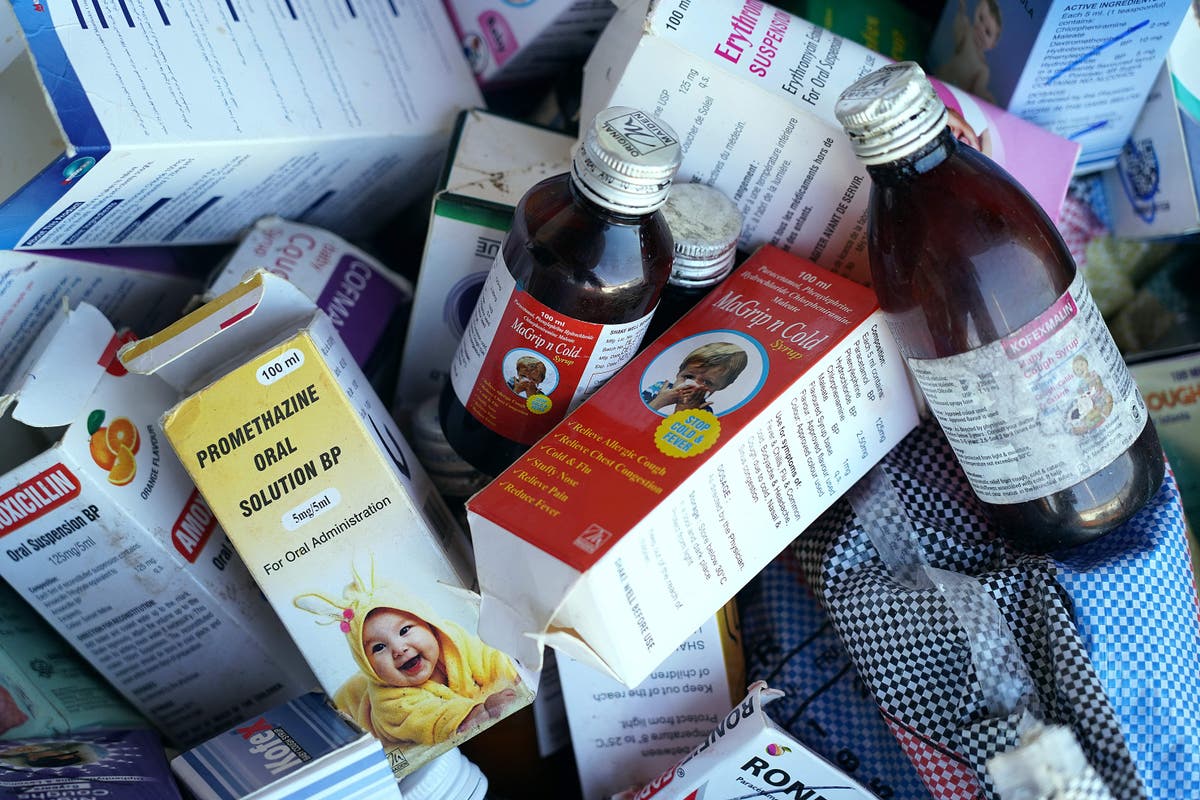
India has ordered its pharmaceutical companies to meet new manufacturing standards this year after a string of deaths reported overseas linked to Indian-made drugs since 2022.
Following deaths linked to India-made cough syrups in countries including Gambia and Uzbekistan, prime minister Narendra Modi’s government increased scrutiny of drug-making factories to restore trust in the country’s image as the “pharmacy of the world”.
…
In 2022, more than 60 children, most under five years of age, died as a result of acute kidney injuries which were linked to cough syrups made in India.
In the same year, 19 children in Uzbekistan who consumed cough syrups made in India died.
Then last year, the manufacturer of over two dozen varieties of eyedrops was subject to a US safety warning officially recalling the products.
The US’s FDA had warned consumers not to use the products due to the risk of vision loss or blindness.
The WHO also flagged a batch of a “contaminated” cough syrup made in India and found in the Marhsall Islands and Micronesia in 2023.


I out India quality below China and I once had an argument with a Chinese manufacturer about the soec requiring Stainless 304 and he used iron and stated his iron is the best iron in China and never rusts.