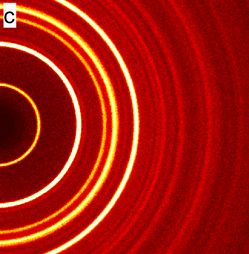
I purchased a pocket-gamma spectrometer last week (Radiacode 102) and brought it with me to a local flea market to find some radioactive antiques! I also found a radioactive gimbaled compass in a box, but did not buy that one.
Here is the processed Gamma spectrum measured with the Radiacode:

Photo with lights on:

Radiacode placed against it:

Pretty rad!
(Pun?)
deleted by creator
deleted by creator
I have very little knowledge about radiation, is this safe?
These are safe to have around because the quantities of Radium in them are very low.
But radium, in large quantities, can very detrimental to one’s health. Around the 1920s people did not know this and they would use it liberally to paint clocks, compassess, signs, and other objects. This was mainly a problem for the workers who were constantly exposed. An important example were the Radium girls: https://en.m.wikipedia.org/wiki/Radium_Girls
There were also a lot of pseudoscientific claims about Radium being good for one’s health, and people would drink drinks with radium in them.
Now we understand that radium, when ingested, can accumulate in the bones where it replaces calcium. When it decays, it and its decay products emit high-energy particles that can kill cells and cause mutations, potentially leading to cancer.
If I’m seeing the meter correctly it’s about 30 cps or 1800 cpm. If it were at a nuclear plant we would consider it radioactive material and wouldn’t let it leave. But it’s certainly far below any harmful level. Source: Former Radiation Protection Manager.
That is extremely cool!
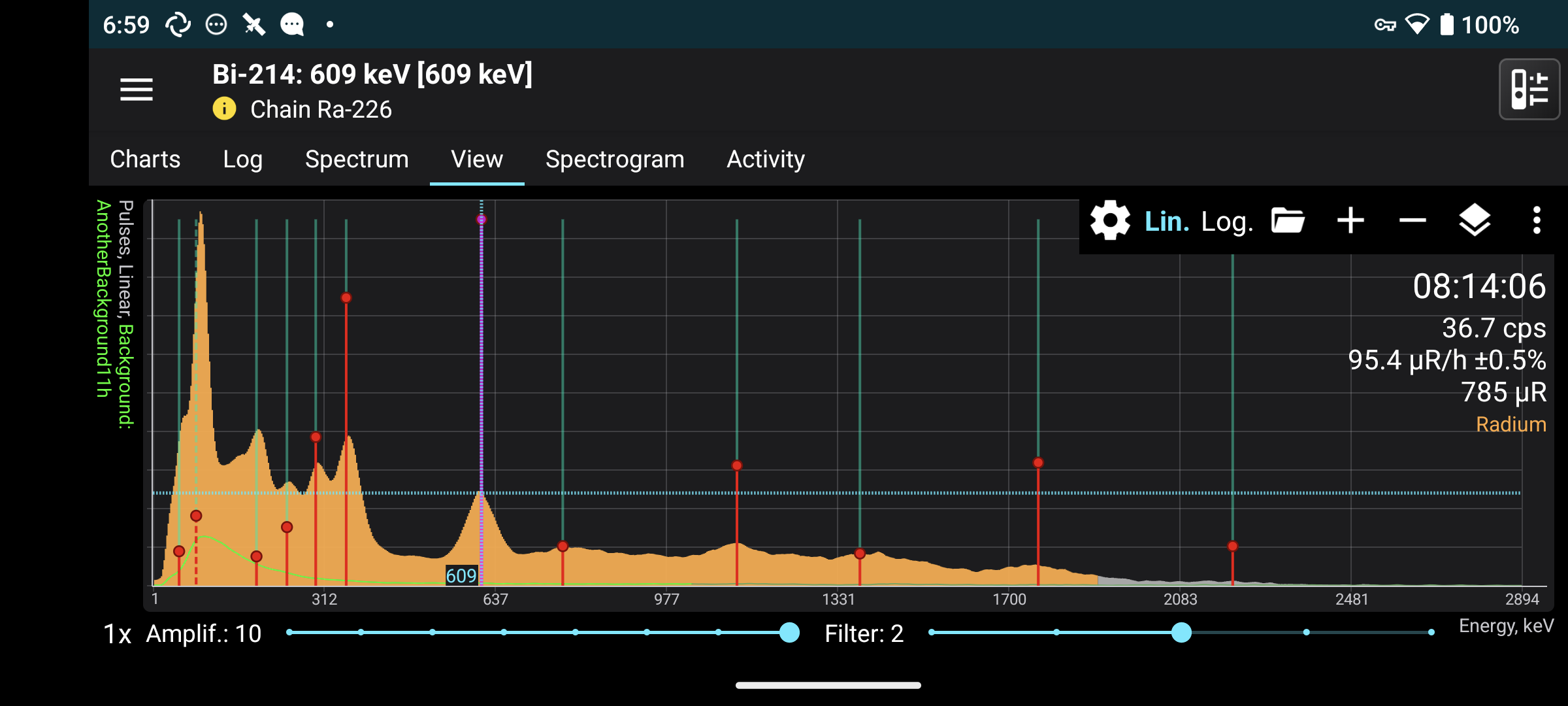
Can you tell which radioactive elements are in it from the spectrum?
Yes, the gamma emission peaks correspond to specific transitions of excited nuclear states or to processes such as the annihilation of an electron and a positron.
The app has a built-in database of common decay chains, so I can click on a peak and lines are drawn at the positions that correspond to the radioactive chain. In this spectrum one can see primarily the lines from the decay of radium-226 and its products lead-214 and bismuth-214
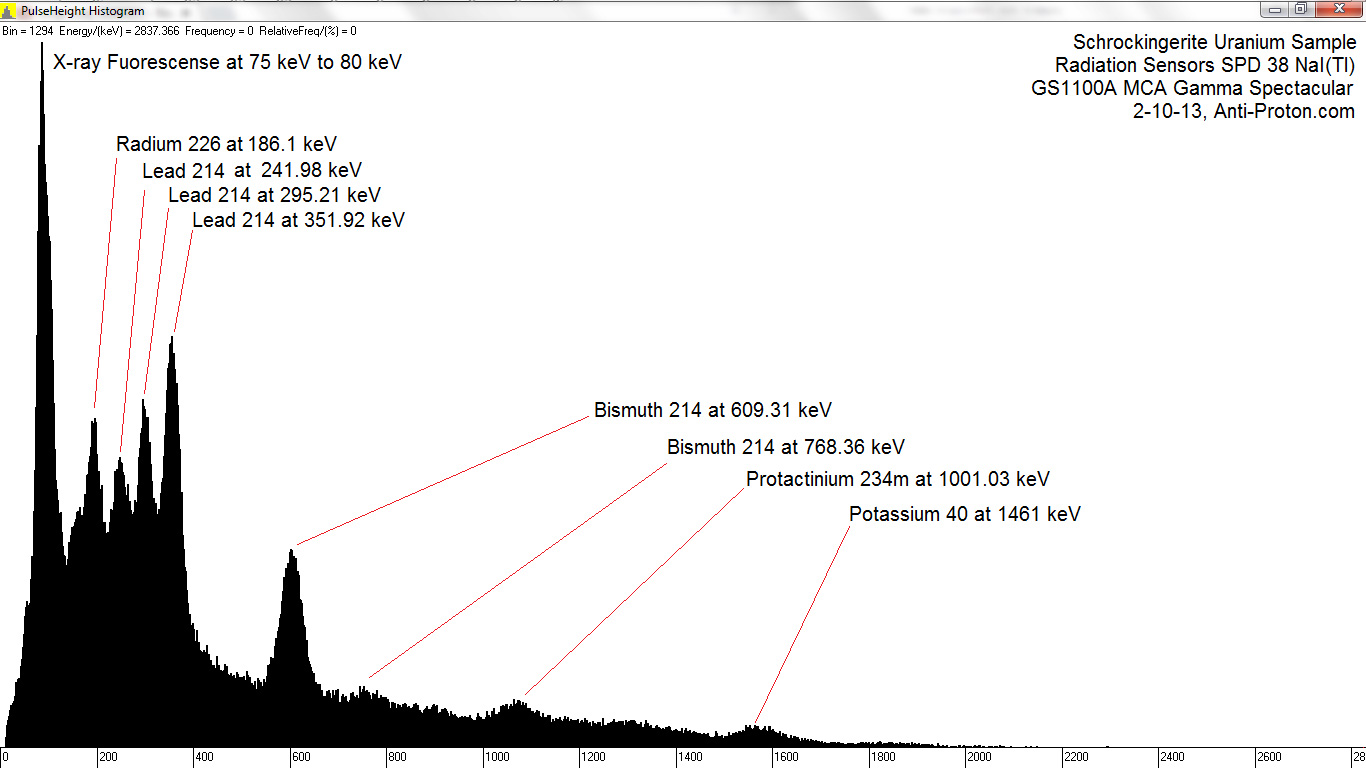
Here is a labeled spectrum I found at https://www.gammaspectacular.com/blue/ra226-spectrum :
awesome